When you hear about molecules in interstellar space or on the moons of Saturn they tend to be small molecules like methane, ammonia or water. A big organic molecule would be something like glycine, the simplest amino acid, with only 5 "big" atoms (carbon, oxygen and nitrogen) and 5 Hydrogen atoms. So finding buckyballs with 60 or 70 carbon atoms is really quite extraordinary. It's a big difference, and buckyballs contain only carbon atoms - no other elements not even hydrogen, the most common element in the universe.
 |
| Buckminsterfullerene - C60 |
Image via Wikipedia
Using the Spitzer Space Telescope, an international reseach group has recently observed the buckminsterfullerenes C60 and C70 in Tc 1, a young Planetary Nebula (PN) with a white dwarf at the center. The inner region of the nebula is carbon-rich, hydrogen-poor and dusty and this seems to be an important reason that they were able to see buckyballs there - buckyballs need lots of carbon in order to form, and they don't have any hydrogen in them at all.
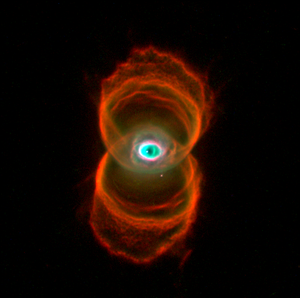 |
| The Hourglass Nebula |
Image via Wikipedia
Most planetary nebulae have strong emissions from polycyclic aromatic hydrocarbons (PAH's) but not Tc 1. Also missing, there are almost no simple hydrogen-containing molecules like HCN or C2H2. A PAH would be like a small piece of a buckyball with hydrogens around the outside edge. Once a buckyball started to form if there was any hydrogen around, hydrogen atoms could attach to the carbons on the edges resulting in a PAH instead of a buckyball.
from the research article:
On Earth, fullerenes can be synthesized by vaporizing graphite in a hydrogen-poor atmosphere that contains helium as a buffer gas. The fullerene formation process is very efficient, and C60 is by far the dominant and most stable species among the large cluster population formed in these experiments, followed by C70. However, fullerene formation is inhibited by the presence of hydrogen. The circumstellar environment of Tc 1 seems to be the astrophysical analog of such a laboratory setup.They used Infra-Red (IR) spectroscopy to identify the buckyballs. It's especially useful in this context because it tells you what kinds of bonds there are in a molecule. Visible light doesn't usually give a lot of useful information except for individual atoms, or maybe certain types of large, complex molecules. But IR gives you a lot of information about the bonds in a molecule. IR spectra can be quite complex - the nifty thing with C60 is that for as large as it is, it has a very simple IR spectrum.
I had a difficult time finding an IR spectrum for C60 at the usual online chemistry databases (NIST Webbook, ChemSpider, SDBS). But this web page has a small image of the IR spectrum of C60. It's a very simple spectrum with just 4 absorptions. The molecular structure of C60 it looks intimidating, but it turns out that there are really only two kinds of bonds: bonds that are shared between two 6-membered rings, and bonds shared between a 5-membered ring and a 6-membered ring. And the thing about IR spectroscopy is that it is highly dependent on symmetry. C60 is highly symmetrical, so you only see 4 absorptions for it. C70 is the second most common buckyball. It's not as symmetrical as C60, and as a result its spectrum is more complex that that of C60.
Cami, J., Bernard-Salas, J., Peeters, E., & Malek, S. (2010). Detection of C60 and C70 in a Young Planetary Nebula Science DOI: 10.1126/science.1192035






No comments:
Post a Comment