Tuesday, November 2, 2010
Jmol test in Blogger
Here is a short test to see if I can display a Jmol applet in a blog post. I don't currently have access to a server to host the jmol applet, so for this test I am linking to the applet, script and molecule files on the Jmol Samples page.
UI Controls example
Friday, October 22, 2010
Chiral Drugs on the Chemistry Blog
Yesterday we started Stereochemistry in my Organic Class, and on Monday Azmanam on the Chemistry Blog had a post on Chiral Drugs. Take a look at the structures below:
Both are used to treat gastroesophageal reflux desease (GERD, commonly known as acid reflux). Can you see the difference in the structures? Click the image to see a larger version. Prilosec is sold as a racemic mixture: a 50-50 mix of the R and S enantiomers. Nexium contains just one enantiomer - the S isomer. This is an example of a chiral molecule whose stereogenic center is not a carbon, but rather a sulfur. The fourth group attached to the sulfur - the one with the lowest priority - is a lone pair of electrons. Read Azmanam 's post for a good discussion of the two.
Chemistry Blog - Nexium’s Dirty Little Secret
Both are used to treat gastroesophageal reflux desease (GERD, commonly known as acid reflux). Can you see the difference in the structures? Click the image to see a larger version. Prilosec is sold as a racemic mixture: a 50-50 mix of the R and S enantiomers. Nexium contains just one enantiomer - the S isomer. This is an example of a chiral molecule whose stereogenic center is not a carbon, but rather a sulfur. The fourth group attached to the sulfur - the one with the lowest priority - is a lone pair of electrons. Read Azmanam 's post for a good discussion of the two.
Chemistry Blog - Nexium’s Dirty Little Secret
Tuesday, September 7, 2010
How Plants Use Caterpillar Spit for Protection
How do plants protect themselves from the bugs that chew on their leaves? In the case of the wild tobacco Nicotiana attenuata, when tobacco hornworm (manduca sexta) caterpillars feed on the leaves a collection of molecules called Green Leaf Volatiles (GLV's) is released by the plant. GLV's are released any time a leaf is damaged, but the interesting thing is that when the damage is done by chewing caterpillars, a different form of the GLV's are produced which attracts Big-Eyed Bugs (Geocoris spp) - a predator for the caterpillars.
 Image via Wikipedia
Image via WikipediaPlants emit two main types of volatile molecules: terpenoids and Green Leaf Volatiles. The terpenoids are emitted from the whole plant and usually after a delay - maybe as much as a day after the damage. The green leaf volatiles are more specific - they are emitted from the damaged leaf itself and it looks like they are produced at the same time as the damage.
Green Leaf Volatiles are typically 6-carbon alcohols, aldehydes or esters. In the case of Nicotiana Attenuata they seem to mostly consist of hexenal, hexenol and simple esters of hexenol. The interesting bit is the alkene portion of these molecules. Alkenes can have one of two basic geometries around the double bond: the Z (or cis) isomer is locked into a u-turn shape and the E (or trans) isomer is locked into a zigzag-like orientation.
Normally, Nicotiana attenuata produces mostly the Z isomer of these molecules and a relatively small amount of the E isomer. However something unusual happens when the damage is caused by caterpillars chewing on the leaves: in this case the plant produces roughly equal amounts of the Z isomer and the E isomer. You and I would probably not notice a difference in the smell of the leaves, but apparently there are bugs that can. When more E isomer is produced, more Big-Eyed Bugs are attracted to the plants. And the big-eyed bug eats caterpillars and their eggs. The E isomer GLV's are a plant distress call and the big-eyed bugs are the cavalry.
How exactly does the plant "decide" which GLV isomers to make? After testing a variety of possible candidates, it looks as though there is an enzyme in the caterpillars' saliva that causes the Z isomers to isomerize to the corresponding E isomers. It is the caterpillar spit that produces the distress call.
If you look closely at the Z molecules and the E molecules you will notice that there are actually two changes that take place. First, the geometry around the alkene switches. In general, the E isomer is more spread-out than the Z isomer and as a result it is lower in energy. Given a choice the alkene will usually adopt the E geometry. If there is a catalyst available, this change is pretty easy to understand.
The second thing that changes is the location of the alkene, the alkene moves closer to the oxygen end of the molecule. Enzymes are very efficient molecules and they are very sensitive to shape. My guess is that the "real" target for the isomerase in the caterpillar saliva is the aldehyde. The aldehyde has a carbonyl group as well as the alkene and the most stable arrangement for these two functional groups is the one in hex-2-enal. When the two double bonds are separated by only one single bond their orbitals are able to interact and form a conjugated system. The conjugated version is more stable than the one where the two double bonds are farther apart and unable to interact with one another.
If improved conjugation in the product is the reason that the alkene moves from the 3-position to the 2-position, why does the alkene move in the alcohol and ester molecules too? The alcohol has only one double bond since there is no C=O, so conjugation is not possible in this molecule. And while the ester does have a C=O, it is too far away to interact with the 2-alkene to form a conjugated system. What gives?
Enzymes can be very selective about the molecules that they react with, but they can also be forgiving if the structure is not exactly correct. A lot of drugs affect specific enzymes in the body - the drug isn't exactly the correct shape, but it's close enough to bind to the enzyme. In the case of the GLV's, the alcohol and ester molecules are close enough to the right shape to bind to the enzyme and react. In the aldehyde the enzyme causes the alkene to migrate as well as change shape because it forms conjugated molecule. Even though the alcohol and ester don't benefit from forming a product molecule that has conjugation, the enzyme treats them the same way it treats the aldehyde and the alkene migrates to the 2-position.
The other curious thing about this is the isomerase enzyme in the caterpillar saliva. I would bet the reason the caterpillars make this enzyme has nothing to do with attracting big-eyed bugs to come eat the caterpillars, that would be counter productive. The plants probably evolved their GLV's to take advantage of this enzyme that the caterpillars make anyway. So what is the isomerase "supposed" to do that benefits the caterpillars?
The smell of freshly-cut grass is actually a plant distress call | IO9.COM
Allmann S, & Baldwin IT (2010). Insects betray themselves in nature to predators by rapid isomerization of green leaf volatiles. Science (New York, N.Y.), 329 (5995), 1075-8 PMID: 20798319
Sunday, September 5, 2010
How to safely put your hand into really scary liquids - fun with the Leidenfrost effect
 Image via Wikipedia
Image via WikipediaCheck out these two videos demonstrating the Leidenfrost effect. If you have ever seen drops of water bounce around on a hot skillet, that's the Leidenfrost effect.
First Theo Gray puts his hand into liquid Nitrogen. Liquid Nitrogen is really cold: −196 °C, −321 °F. You have probably seen demonstrations where something like a rubber ball or a rose is dipped in Liquid Nitrogen - on freezing at such a low temp most things will shatter if dropped or hit with a hammer.
Theo Gray dips his hand into a large container of liquid Nitrogen without developing a permanent case of frost bite by taking advantage of the Leidenfrost Effect. Since his hand is much warmer than the liquid nitrogen, a very thin layer of gaseous nitrogen forms and acts as a protective barrier between the bulk liquid nitrogen and the surface of his hand.
Adam and Jamie demonstrated the same effect with molten lead on an episode of Mythbusters. This is kind of the opposite of the liquid nitrogen case - instead of using an extremely cold liquid they are using a very hot liquid. Lead melts at 621 °F, but they actually did the experiment at about 800 °F.
To be protected by the Leidenfrost effect they needed a thin layer of gas between their hands and the lead, so they dipped their hands in water and shook off the excess before putting their hands into the liquid lead. The small amount of water on their hands vaporized to provide the thin, protective layer of gas between their skin and the liquid lead. The fun starts about 2 minutes into the clip.
It goes without saying - making a mistake when doing this will have severe consequences. Don't try this at home.
You can safely stick your hand in liquid nitrogen...but you probably shouldn't | IO9.com
Friday, September 3, 2010
Laboratory Disaster Stories
Do you need some good reasons to wear your lab goggles? Check out the Lab Horror Stories thread on Reddit.
 Image via Wikipedia Here's one short and sweet example from an organic chemistry lab:
Image via Wikipedia Here's one short and sweet example from an organic chemistry lab:
Link from Boingboing:
Or does it explode?: Reddit collection of laboratory disaster stories - Boing Boing
 Image via Wikipedia
Image via WikipediaWhen I was in organic lab, my TA closed my heating reaction flask a little too tightly. It blew up. I pulled three pieces of glass out of my forehead right above my right eyebrow. The stopper hit my partner in the head. We lived long enough for the department to let us graduate.For educational and entertainment purposes only, please don't do any of these things yourself.
Yay for goggles!
Link from Boingboing:
Or does it explode?: Reddit collection of laboratory disaster stories - Boing Boing
Wednesday, August 11, 2010
ChemSketch on Linux Again
Last year I wrote about using ChemSketch on Linux. It is about the only Windows program that I regularly use these days. Since switching to Linux I have not completely settled on a chemical structure drawing program. Sometimes I still use ChemSketch on Windows and then copy the image into OpenOffice, sometimes use another program on Linux - usually MarvinSketch.
Thanks to a comment left by Dragly on my original ChemSketch post in January, I can now reliably use ChemSketch on Linux.
ChemSketch is a Windows program, so I have to use Wine in order to run ChemSketch. The problem was that ChemSketch would run fine the first time it is started after installation, but every other time I tried to run the program it would hang-up without the ChemSketch window ever appearing.
Dragly's comment referred me to:
After digging around a bit, it turns out that the problem is in the Wine registry file, which for me is at
The clever thing to do would be to edit user.reg to delete the offending setting, which starts with
Under Windows, you can open the 3DViewer from ChemSketch by using the ACD/Labs menu. Unfortunately, when I run under Linux, this menu does not have any of the labels visible. The first menu item is the 3DViewer. Or you can right-click the ACD/Labs icon in the Gnome panel at the top of the screen and select the 3D Viewer to open it.
Copying between the ChemSketch and 3D Viewer windows works, and so do the Database search options. The only remaining problem is getting the figure into an Open Office document. You can't simply copy the image, nor can you insert an OLE object the way you would in Windows. Instead you will need to save the figure as an image and import the image into the Open Office document. The downsides of this are adding an extra step to save the image, and not being able to edit the image easily unless you also saved the original ChemSketch file.
Thanks to a comment left by Dragly on my original ChemSketch post in January, I can now reliably use ChemSketch on Linux.
ChemSketch is a Windows program, so I have to use Wine in order to run ChemSketch. The problem was that ChemSketch would run fine the first time it is started after installation, but every other time I tried to run the program it would hang-up without the ChemSketch window ever appearing.
Dragly's comment referred me to:
http://markmail.org/message/grddjlgsn3dh5kqpAnd he also pointed out that the program could be run with the window maximized by using this command, provided that the file path to the program is correct:
wine start /MAX C:\\WINDOWS\\TEMP\\ACDFREE12\\CHEMSK.EXEThis works, but it behaves a bit flakey for me running Ubuntu 10.04 and Wine 1.1.42. First the ACD/Lab Products panel appears (very slowly) - after clicking the "OK" button, the program itself loads on the workspace to the right of the one I am working on. The 3D View program behaves the same way - you need to use:
wine start /MAX C:\\WINDOWS\\TEMP\\ACDFREE12\\SHOW3D.EXEto get it to open normally, and then it shifts one workspace to the right.
After digging around a bit, it turns out that the problem is in the Wine registry file, which for me is at
/home/steve/.wine/user.regThis file is updated every time you close a program that is running under Wine. The quick and dirty solution would be to delete this file before running ChemSketch. Except for losing all the settings for every program you run with Wine, this works pretty well. The program starts normally - if a bit slowly compared to Windows.
The clever thing to do would be to edit user.reg to delete the offending setting, which starts with
[Software\\Advanced Chemistry Development (ACD)\\Size]and is followed by a bunch of numbers. Deleting this setting allows you to run ChemSketch and the 3DViewer "normally." This is a bit tedious but do-able. To be really clever I should write a program to do it for me every time I run ChemSketch.
Under Windows, you can open the 3DViewer from ChemSketch by using the ACD/Labs menu. Unfortunately, when I run under Linux, this menu does not have any of the labels visible. The first menu item is the 3DViewer. Or you can right-click the ACD/Labs icon in the Gnome panel at the top of the screen and select the 3D Viewer to open it.
Copying between the ChemSketch and 3D Viewer windows works, and so do the Database search options. The only remaining problem is getting the figure into an Open Office document. You can't simply copy the image, nor can you insert an OLE object the way you would in Windows. Instead you will need to save the figure as an image and import the image into the Open Office document. The downsides of this are adding an extra step to save the image, and not being able to edit the image easily unless you also saved the original ChemSketch file.
Tuesday, August 10, 2010
Finding Buckyballs in Space
When you hear about molecules in interstellar space or on the moons of Saturn they tend to be small molecules like methane, ammonia or water. A big organic molecule would be something like glycine, the simplest amino acid, with only 5 "big" atoms (carbon, oxygen and nitrogen) and 5 Hydrogen atoms. So finding buckyballs with 60 or 70 carbon atoms is really quite extraordinary. It's a big difference, and buckyballs contain only carbon atoms - no other elements not even hydrogen, the most common element in the universe.
 |
| Buckminsterfullerene - C60 |
Image via Wikipedia
Using the Spitzer Space Telescope, an international reseach group has recently observed the buckminsterfullerenes C60 and C70 in Tc 1, a young Planetary Nebula (PN) with a white dwarf at the center. The inner region of the nebula is carbon-rich, hydrogen-poor and dusty and this seems to be an important reason that they were able to see buckyballs there - buckyballs need lots of carbon in order to form, and they don't have any hydrogen in them at all.
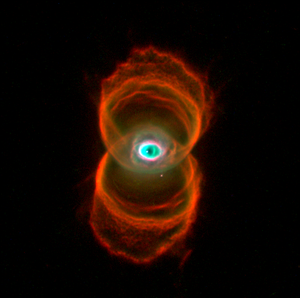 |
| The Hourglass Nebula |
Image via Wikipedia
Most planetary nebulae have strong emissions from polycyclic aromatic hydrocarbons (PAH's) but not Tc 1. Also missing, there are almost no simple hydrogen-containing molecules like HCN or C2H2. A PAH would be like a small piece of a buckyball with hydrogens around the outside edge. Once a buckyball started to form if there was any hydrogen around, hydrogen atoms could attach to the carbons on the edges resulting in a PAH instead of a buckyball.
from the research article:
On Earth, fullerenes can be synthesized by vaporizing graphite in a hydrogen-poor atmosphere that contains helium as a buffer gas. The fullerene formation process is very efficient, and C60 is by far the dominant and most stable species among the large cluster population formed in these experiments, followed by C70. However, fullerene formation is inhibited by the presence of hydrogen. The circumstellar environment of Tc 1 seems to be the astrophysical analog of such a laboratory setup.They used Infra-Red (IR) spectroscopy to identify the buckyballs. It's especially useful in this context because it tells you what kinds of bonds there are in a molecule. Visible light doesn't usually give a lot of useful information except for individual atoms, or maybe certain types of large, complex molecules. But IR gives you a lot of information about the bonds in a molecule. IR spectra can be quite complex - the nifty thing with C60 is that for as large as it is, it has a very simple IR spectrum.
I had a difficult time finding an IR spectrum for C60 at the usual online chemistry databases (NIST Webbook, ChemSpider, SDBS). But this web page has a small image of the IR spectrum of C60. It's a very simple spectrum with just 4 absorptions. The molecular structure of C60 it looks intimidating, but it turns out that there are really only two kinds of bonds: bonds that are shared between two 6-membered rings, and bonds shared between a 5-membered ring and a 6-membered ring. And the thing about IR spectroscopy is that it is highly dependent on symmetry. C60 is highly symmetrical, so you only see 4 absorptions for it. C70 is the second most common buckyball. It's not as symmetrical as C60, and as a result its spectrum is more complex that that of C60.
Cami, J., Bernard-Salas, J., Peeters, E., & Malek, S. (2010). Detection of C60 and C70 in a Young Planetary Nebula Science DOI: 10.1126/science.1192035
Sunday, August 1, 2010
The Chemistry of Cthulhu?
I haven't posted in a while - is this the reason?
Was I tottering on the brink of cosmic horrors beyond man's power to hear?
I suppose that some people might say of Organic Chemistry that
... there is no language for such abysms of shrieking and immemorial lunacy, such eldrich contradictions of all matter, force, and cosmic order.
Image via Wikipedia
Tuesday, May 4, 2010
Thinking about Simplicity
Harvard Organic Chemistry professor George Whitesides takes a stab at defining "simplicity." Here's a wonderful, thoughtful talk for the end of the school year.
Monday, February 8, 2010
Top 30 Science Blogs at Times Online - Eureka Zone
The Eureka Zone science blog at the NY Times has a list of their top 30 science blogs. If you are looking for science reading this is a great place to start. I only wish there was a Chemistry blog or two on their list.
Times Online - Eureka Zone: Eureka's Top 30 Science Blogs
Times Online - Eureka Zone: Eureka's Top 30 Science Blogs
Saturday, February 6, 2010
Friday, February 5, 2010
George Whitesides Discusses Designing a "Lab on a Stamp"
This talk is listed at the Ted.com site as "A Lab the Size of a Postage Stamp," but it should really be "A ON a Postage Stamp." He talks about the ingenious way his group has designed "devices" for medical diagnostics from paper. This makes it very inexpensive as well as easily disposable - no sharps or bio-waste to worry about, you can just burn the device when you are done.
George Whitesides is an Organic Chemist from Harvard - I would love to hear him talk about his work on Self-Assembly, but this is pretty cool too. To quote from his Bio at Ted:
He's co-founded a nonprofit called Diagnostics for All that aims to provide dirt-cheap diagnostic devices, to provide healthcare in a world where cost is everything.While sharing some of his experience, he also has some interesting observations of the nature of our society, given that the cost of healthcare has been such a topic of discussion lately.
Sunday, January 17, 2010
Moebius Strip Bach Canon
A little MatheMusical fun for Sunday.
via BoingBoing Bach canon played as a moebius strip - Boing Boing
Monday, January 11, 2010
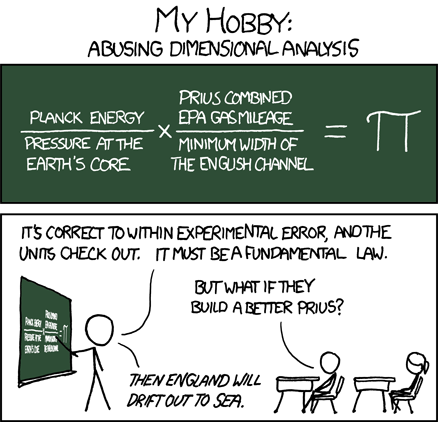
Fun With Dimensional Analysis
It's amazing what you can do if you can just get the units to cancel.
Subscribe to:
Posts (Atom)